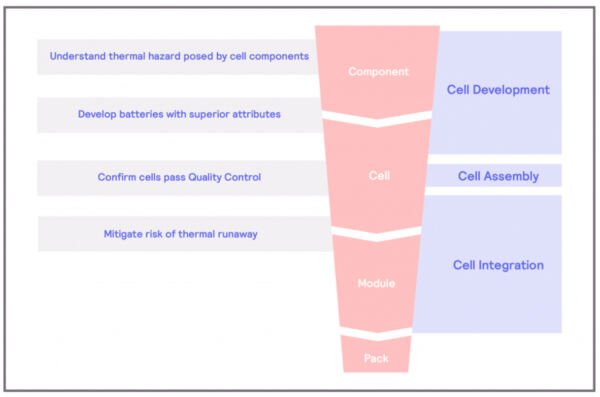
Thermal Hazard of Component
What are the thermal hazards of the cell components?
The main components of an electrochemical cell – the basic component of a battery – are the anode, the cathode, and the electrolyte. The main sources of concern in electrical batteries are the electrolyte and the cathode. Organic carbonates are commonly used as electrolytes and are susceptible to decomposition reactions. These reactions involve a molecule breakdown, resulting in two or more chemical compounds. These reactions are typically exothermic, and energy is released in the process, which further fuels the breakdown of chemical compounds, creating a chain reaction called a thermal runaway. In the case of the decomposition of electrolytes, the results of the reactions are typically gases, some of which can be flammable. Decomposition reactions in batteries are normally evidenced by the compromise of the structural integrity of the battery itself.
On the other hand, cathodes are usually composed of a metal oxide, such as LiCoO2. When exposed to high temperatures, like those resulting from a thermal runaway, these metal oxides can undergo decomposition. The result of this decomposition will be the metal plus oxygen.
This is where the main problem of Lithium-ion batteries lies. The combination of flammable gases and oxygen and the extra heat provided can result in fire and battery explosions.
The separator is another component of the cells that can be susceptible to thermal hazards. Separators are designed to act as a physical barrier between the anode and cathode should a malfunction occur in the battery, preventing short-circuit events. However, most separators nowadays are made from polymers, which, in some cases, can melt or shrink at high temperatures. If this event takes place, thermal runaways could happen.
Solutions
Adiabatic calorimeters are designed to prevent heat exchange between the battery and its surroundings. Batteries experience shifting conditions during their lifespan, including heating and cooling. It is fundamental to understand how the cell components behave under a wide range of temperatures and environments and if they are susceptible to thermal runaways. An adiabatic calorimeter simulates a worst-case scenario where the battery is unable to dissipate its heat while under operation. BTC-130 is a bench-top adiabatic calorimeter especially designed for component and cell testing, including thermal, electrical, and mechanical stress. Using the BTC-130, you can assess the safety performance of the battery cell, the safe operating limit, and the consequences of the thermal runaway.
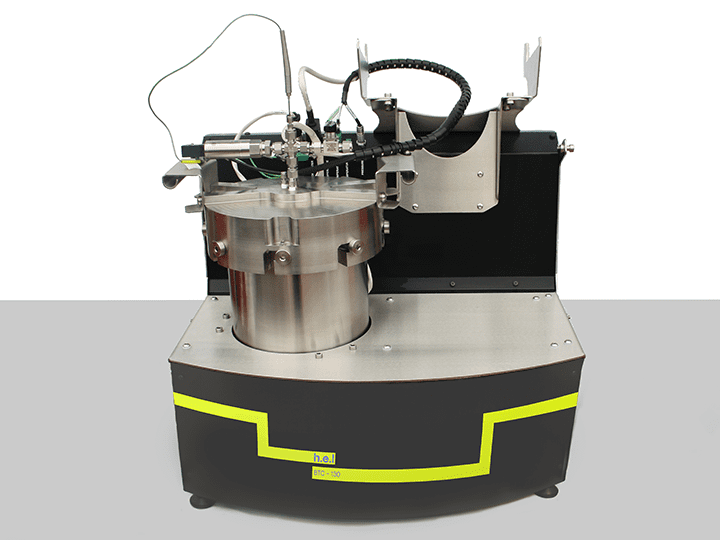
BTC-130 | Bench-Top, Battery Safety Testing, Adiabatic Calorimeter
The BTC-130 (Battery Testing Calorimeter) is a bench-scale adiabatic calorimeter designed ...