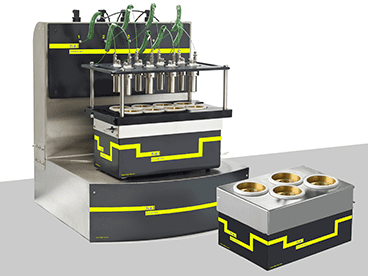
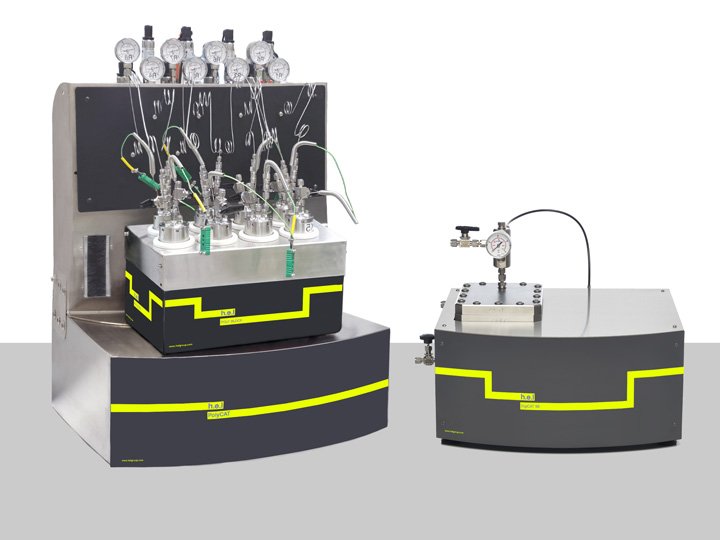
Catalysis and Hydrogenation
H.E.L Group’s range of catalysis and hydrogenation solutions provides powerful reaction screening and analysis in a benchtop package designed to give insights into high-pressure catalysis at the research, process development, or scale-up stage, with support for batch processing, parallel analysis, and continuous flow reactions.
Automation, process control, safety monitoring, and data capture through the labCONSOL platform gives you the confidence to run your high-pressure catalysis experiments efficiently and effectively. This results in the key insights you need on your reactions to optimize your chemistry to produce the quality, quantity, and purity you need, along with actionable reaction information.